Pour répondre à des exigences essentielles de santé et de sécurité, le marquage CE a été créé dans le cadre de la législation d’harmonisation technique européenne. Il reconnait qu’un produit peut être vendu dans l’union européenne et qu’il respecte les normes et réglementations associées. Le milieu de la santé et des dispositifs médicaux ne déroge pas à cette règle. Pour pouvoir déployer un dispositif médical dans l’UE, un fabricant doit obtenir le marquage CE.
Qu’est-ce qu’un dispositif médical ?
« Tout instrument, appareil, équipement, logiciel, implant, réactif ou autre article, destiné par le fabricant à être utilisé, seul ou en association, chez l’homme pour l’une ou plusieurs des fins médicales précises suivantes :
- diagnostic, prévention, contrôle, prévision, pronostic, traitement ou atténuation d’une maladie,
- diagnostic, contrôle, traitement, atténuation ou compensation d’une blessure ou d’un handicap,
- étude, remplacement ou modification d’une structure ou fonction anatomique ou d’un processus ou état physiologique ou pathologique,
- communication d’informations au moyen d’un examen in vitro d’échantillons provenant du corps humain, y compris les dons d’organes, de sang et de tissus,
et dont l’action principale voulue dans ou sur le corps humain n’est pas obtenue par des moyens pharmacologiques ou immunologiques ni par métabolisme, mais dont la fonction peut être assistée par de tels moyens. »
Article 2 du règlement 2017/745
Notre logiciel engage, par son module « Suivi connecté » et son déclenchement d’alertes, permet la surveillance d’une pathologie, d’une plaie ou d’un handicap. L’action principale voulue dans ou sur le corps humain n’est pas obtenue par des moyens pharmacologiques, immunologiques ni par métabolisme. Il s’agit donc d’un dispositif médical. »
Quelle certification ?
Le marquage CE matérialise la conformité d’un produit aux exigences communautaires incombant au fabricant du produit, en vue de sa libre circulation sur l’ensemble de l’UE.
Le marquage CE médical est basé sur un examen qui garantit que le dispositif médical répond à des exigences spécifiques de sécurité et de bénéfice clinique, fixées par la réglementation européenne (Règlement 2017/745, anciennement Directive 93/45 CEE).
Cette règlementation fait intervenir différents acteurs :
- Le fabricant/éditeur de logiciel
- L’organisme notifié (excepté pour les DM de classe I)
- L’autorité compétente. En France, il s’agit de l’Agence Nationale de la Santé et du Médicament (ANSM).
Quelles conformités ?
Deux étapes sont nécessaires à la mise en conformité des DM :
- Évaluation du DM (dossier technique, conception, données cliniques, production, analyse des risques…) par un organisme notifié lors d’un audit.
- Mise en place d’un système de management de la qualité dans l’entreprise conformément à la norme ISO 13485 et évaluation par un organisme notifié (capacité à produire et de vendre que des produits conformes) lors d’un audit.
Quelles sont les différentes classes de risque des dispositifs médicaux ?
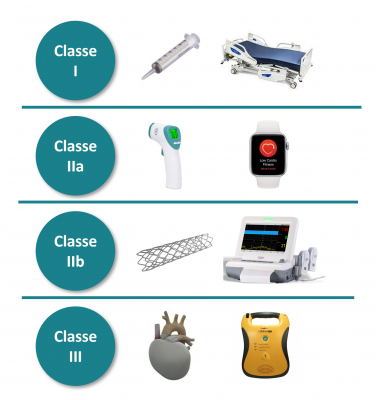
La classe d’un dispositif médical est importante car elle permet de définir les exigences réglementaires applicables à un DM et aux activités de son fabricant. Elle est déterminée en fonction de ses caractéristiques et des règles déterminées dans le règlement 2017/745 et à la dangerosité du dispositif. Dans ces caractéristiques prédéfinies, on y retrouve :
- La durée d’utilisation
- Le type de DM
Les réponses apportées à ces exigences définissent la classe de sécurité du DM. Elles ont un impact direct sur la certification, car plus la classe de risque est élevée et plus les contraintes réglementaires sont grandes. Il existe ainsi quatre classes pour les DM, par ordre de criticité : I / IIa / IIb / III
La criticité est déterminée en fonction du potentiel risque pour le patient, le personnel soignant ou toute autre personne intervenant lors de l’utilisation du dispositif.
Quelle importance pour exolis ?
Comme précisé auparavant, notre logiciel engage est un dispositif médical grâce à son module « suivi connecté », sa gestion des alertes, ses questionnaires et l’envoi des réponses aux personnels soignants. C’est pourquoi, nous réalisons les démarches nécessaires afin de conformer notre logiciel :
- Depuis mai 2021, engage est un dispositif médical de classe I, marqué CE selon la règlementation applicable.
- Depuis juillet 2022, exolis est certifié ISO 13485.
Afin de rester conforme aux exigences règlementaires, exolis réalise le maintien à jour de ses dossiers techniques.
Concernant la marque blanche :
Pour permettre un paramétrage spécifique à chaque établissement de santé utilisateur, nous déployons notre application en marque blanche. Les solutions « filles » créées conservent le marquage CE.

Article rédigé par :
Maxime GACHIE, Responsable affaires règlementaires chez exolis by HOPPEN





