Qu'est-ce que la qualité ?
La qualité désigne, selon l’ISO 9000, “l’aptitude d’un ensemble de caractéristiques intrinsèques d’un objet à satisfaire des exigences”. Il est alors possible de définir la qualité d’un produit, d’un service, des soins apportés aux personnes, etc.
Dans l’industrie du dispositif médical, la qualité concerne l’aptitude d’un dispositif à satisfaire :
- les exigences des clients,
- les exigences imposées par la règlementation,
- les exigences relatives à l’environnement dans lequel s’inscrit le dispositif
- les exigences liées à la sécurité du patient ou de l’utilisateur du dispositif.
Ces exigences peuvent être des obligations ou des besoins énoncés ou induits par ces entités.
Pourquoi mettre en place une démarche qualité ?
Ce que l’on cherche avant tout, en tant qu’industriel, c’est la satisfaction client. Pour y parvenir, il est important de pouvoir identifier les exigences du client, de savoir les implémenter dans nos produits et d’avoir la capacité de mesurer cette satisfaction pour évaluer notre aptitude à identifier et implémenter ces exigences.
L’identification de ces trois éléments constitue finalement la base d’une démarche qualité. À cela viendra s’ajouter des méthodes de traçabilité, de documentation et d’amélioration de nos activités.
La démarche qualité s’oriente principalement autour de trois axes :
- La compétitivité : par la recherche et le développement, le développement durable, l’externalisation d’activités, etc.
- L’organisation : par le tri, le contrôle, l’amélioration, etc.
- L’adaptabilité : par la conquête de nouveaux marchés, la connaissance de l’environnement règlementaire, la connaissance du marché, etc.
Le système de management de la qualité
Le management de la qualité au sein des organisations est normalisé par la série des normes ISO 9000 parmi lesquelles se trouve l’ISO 9001 qui décrit les exigences relatives aux systèmes de management de la qualité. Ces systèmes visent non seulement à assurer la qualité des produits et services distribués par une organisation, mais également à promouvoir l’amélioration continue de ces produits et services, de l’organisation et du système de management de la qualité lui-même !
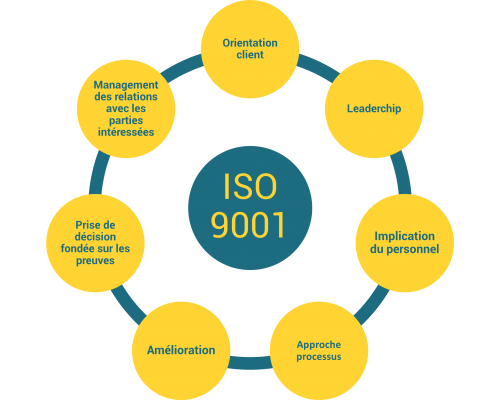
Un système de management de la qualité se base sur 7 principes définis dans l’ISO 9001 :
La qualité en santé
Dans notre secteur, la qualité est un impératif tant règlementaire qu’éthique. Si la règlementation, en l’espèce du Règlement UE 2017/745 relatif aux dispositifs médicaux, impose la mise en place d’un système de management de la qualité, il s’agit en premier lieu d’assurer l’efficacité des soins apportés aux patients. On cherche, par cette démarche qualité, à s’assurer que les produits, services, médicaments, employés pour améliorer la condition de santé des patients atteignent effectivement les objectifs, mais également n’engendrent pas d’effets indésirables imprévus.
La norme ISO 13485 apporte des exigences supplémentaires par rapport aux critères de la série des normes ISO 9000 applicables, notamment aux industriels du secteur des dispositifs médicaux. Sa mise en application tout comme pour l’ISO 9001, est sanctionnée par un cycle renouvelable de trois audits annuels réalisés par un organisme certificateur donnant lieu à la délivrance d’un certificat ISO. Pour les dispositifs de classe supérieure à la classe I, c’est l’organisme notifié qui est chargé de cette tâche et un quatrième audit inopiné vient s’ajouter au cycle.
La norme ISO 13485 est d’application volontaire, c’est-à-dire qu’il n’y a pas, en soi, d’obligation règlementaire à suivre cette norme. Cependant, la mise en place et le maintien d’un système de management de la qualité est une obligation et l’audit de ce système l’est également pour tous les dispositifs de classe supérieure à la classe I.
La qualité chez exolis
Chez exolis, nous avons fait le choix d’appliquer la norme ISO 13485 et de faire certifier notre système de management de la qualité par l’Afnor, l’organisation française qui représente la France auprès de l’ISO (International Organization for Standardization, l’organisation internationale responsable de la production des normes ISO).
Notre système de management de la qualité couvre l’ensemble de nos activités, qu’elles concernent ou non le module de suivi connecté de la solution engage (module dispositif médical). Ce choix nous permet d’apporter un gage de qualité auprès de nos clients et d’engagement à leur fournir des produits et services en constante amélioration, suivant l’état de l’art et conforme aux exigences règlementaires.
L’audit de certification a eu lieu à la fin du premier semestre de l’année 2022 et le certificat nous a été délivré le 21 juillet 2022. Ce certificat atteste que le système de management de la qualité mis en place par exolis pour les activités de conception, fabrication, mise sur le marché, installation et maintenance de systèmes, de logiciels de planification et d’organisation de parcours de soins, déployé sur notre site d’activité a été évalué et jugé conforme aux exigences requises par la norme ISO 13485 version 2016.
Cet audit a évalué l’organisation interne d’exolis, la qualité de nos processus, notre capacité de suivi et d’amélioration de ceux-ci et la qualité du suivi post-commercialisation de nos produits et services.
Concernant la suite, nous sommes en cours d’implémentation des exigences relatives au référentiel QHN (Qualité Hôpital Numérique), mais cela fera l’objet d’un nouvel article !

Article rédigé par :
Camille MERINI, Responsable qualité et affaires règlementaires chez exolis






